The importance of patient selection strategy: Learning from VelosBio

It's a new year and a slow start here at Bench to Biotech. Over the last several weeks, we've seen COVID-19 vaccines approved and new distribution plans be drafted. There is a lot of concern and misinformation around the new variants and whether the existing vaccines will protect us, so let me know if you'd like a deep dive.
I also helped launch the Accelerator for Cancer Therapeutics and I couldn't be more proud of our inaugural cohort. We're in the middle of bootcamp right now, but I hope that there will be exciting news to share over the course of the year as we continue this new experiment in the well-tested accelerator model.
In the meantime, I thought it might be fun to share a case study from one of our bootcamp sessions, highlighting the importance of patient selection in driving outsized outcomes.
Last November, Merck announced an acquisition of the 3-year old VelosBio for a truly eye popping $2.75 Billion in cash. The pharma industry is known for announcing mega deals with payouts largely dependent on clinical milestones that may fail to materialize, often scathingly referred to as biobucks. So this cash transaction based on a lead asset barely cleared through Phase I trials is worth a closer look.
The timeline
- August 21, 2020 – Phase I trial completed
- August 31, 2020 – Fast Track Designation based on results in Mantle Cell Lymphoma
- October 16, 2020 – Phase II trial initiated in Breast Cancer and Non-Small Cell Lung Cancer
- November 5 , 2020 – Merck acquires VelosBio for $2.75B
The asset: VLS-101
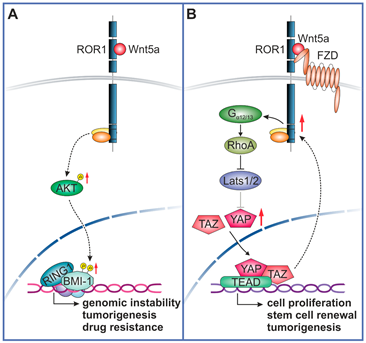
Before we can get to the trials, it's important to understand the context of the lead asset. VLS-101 is an anti-ROR1 antibody-drug conjugate (ADC). By linking a cancer-killing chemotherapy directly to a targeting antibody, you can kill cancer cells while leaving untargeted cells relatively undamaged. The real crux of this technology, then, is dependent on what target you select and it's relative importance to cancer while hopefully having low expression in normal tissues.
Enter ROR1. The ROR1 receptor triggers two key pathways expressed in cancer stem cells and malignant metastasis (tumor spread), while having low expression in normal tissues. However, the WNT pathway families in general have been difficult to target using classic drug methods.
Even as an ADC, it's really important to note that CSC targeting wouldn't be expected to melt tumors away into nothingness. Rather, the hope is that you are killing the root of the tumor by eliminating the cells which ultimately create more tumor cells and seed into distant locations. Measuring efficacy in this field has been historically difficult, making patient selection even more important than it would be already.
Picking a patient population
So as mentioned, the tricky thing with ADCs is that you have to pick a target with a really good differential between your tumor state and your normal state. You would expect this drug to work really well in a population with very high tumor-normal expression. On the other hand, high normal tissue expression can create side effects even worse than normal chemotherapy.
This is, of course, somewhat of a simplification. In the real world you (or your grunts) read papers and look for potential interacting pathways, and do studies to support your hunches. And ROR1 is widely overexpressed in a number of tumors. Still, broadly speaking you would pick tumors more on the left side than the right side of this graph.
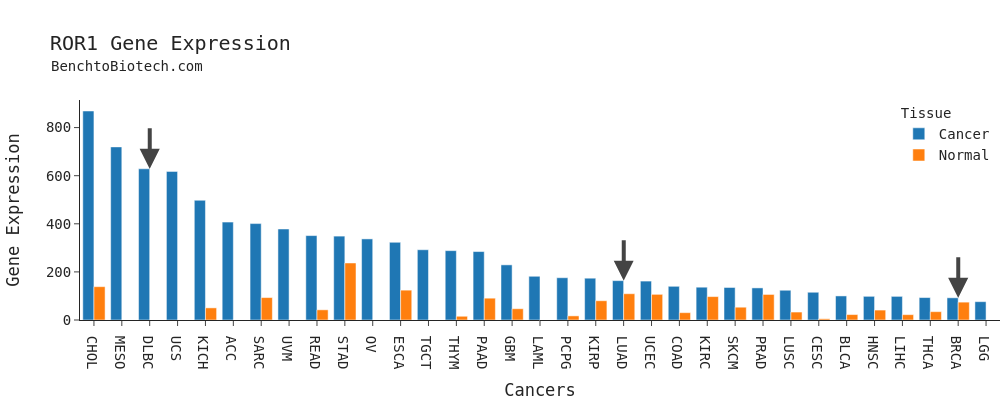
VelosBio planned their Phase I safety studies in the hematological tumors Mantle Cell Lymphoma (MCL) and Diffuse Large B Cell Lymphoma (DLBCL), which is the far left arrow above.
Officially Phase I studies are just to test the safety of a drug, but that doesn't stop your competition, investors, and potential acquirers from judging your likely future success from these 9-15 patients. So picking the right patients can be very very important for getting the opportunity (funds) to go to the next trial.
Mantle Cell Lymphoma is a rare disease even by orphan disease standards. Only 2500-7500 patients are diagnosed with Mantle Cell Lymphoma each year in the USA. But the Phase I results stood out: a striking 80% response rate in DLBC and more modest but important 40% response in the underserved MCL population. The FDA granted Velos a Fast Track Designation in Mantle Cell Lymphoma, the first step in the coveted Accelerated Approval process.
Interestingly, VelosBio had already filed their intended Phase II studies with the FDA. Of course they planned to expand the MCL and DLBCL patient populations. But as they looked to expand to solid tumors, they selected Lung Cancer and Breast Cancer as their starting populations; tumors to the right side of the expression graph. (These are the right two arrows)
This is a notable oddity in a world where "all comers" trials are common and often used to refine further development. Presumably they had excellent preclinical data in hand to support these populations versus any other. But an equally important part of the puzzle may be that these two tumor categories have large subpopulations without other targeted therapies, and have therefore been considered prime ground for cancer stem cell killing therapies.
The Acquisition
So great clinical data leads to a great buyout, right? Not always.
Another key element is that VelosBio had just raised a jumbo $137M Series B round in July. They didn't have to sell to anybody. They were bought, not sold.
We can assume that Merck was not the only pharma company looking at this deal. Cancer stem cells have been a tantalizing target for over a decade, but have thus far not yielded much in the way of clinical results. So a new contender in the field would be worth the price to keep it out of the hands of the competition.
Lessons learned
The point here is not to make you jealous of the beaches and chateaus the VelosBio management is undoubtedly spending their quarantine relaxing at. But rather to drive home the real dollar impact smart patient selection can make in proving the value of a new, potentially wide reaching therapeutic.
Patient selection strategy is the difference between getting to develop a drug and helping patients, or not.
Key takeaways
- Patient selection matters, the earlier the better
- Look for the intersection between biology and market need
- Outsized results in small patient populations can prove your value, so long as you have a path to larger populations
